Chapter 7: Self Regulating Systems -- Atmospheric Gases -- Greenhouse Effect
The behavior of non-living things and systems can be described by applying the laws of physics and chemistry. For example, using the laws of thermodynamics we can calculate what will happen in a melt or solution at a given temperature and pressure (precipitation vs dissolution of minerals), and using the laws of physics we can model the evaporation of ocean waters near the equator, the rising air masses, their flow poleward, and their organization into several belts of convection cells due to the Coriolis force. In all these instances, systems move towards the lowest energetic state, heat moves from warmer to colder regions, energy is dispersed, and equilibrium is achieved (or at least approached). To illustrate this in a simple way, let us look at a metal ball in a bowl.
 |
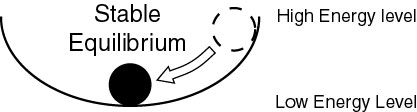
If we place the metal ball in the bowl, the ball will follow gravity and go to the lowest point in the bowl where it finds a state of stable equilibrium. |
 |
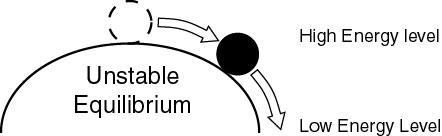
If we turn the bowl upside down, we may be able to balance the ball on top (labile equilibrium), but the slightest disturbance will make it roll down and go to a more stable position at a lower energy level. |
We take it for granted that we can stand upright, that we can stay vertical (more or less) on a rolling ship or on a moving train, and that we don't fall on our faces on a regular basis. The laws of physics don't account for that ability. If it were up to them we would be lying flat on the ground (lowest energy level, center of gravity as close as possible to ground surface). Instead, our center of gravity is about 2-3 feet above the ground and we are basically in a state of unstable equilibrium. The only time we lie flat is when we want to, or when we are so sick that our muscles won't support our weight, or when we are dead.
Th reason why classical physics can not account for our upright walk is that it is most successful in describing and predicting unorganized complexity. An example would be the behavior of a gas as the result of the unorganized and individually untraceable movements of gas molecules. This kind of unorganized complexity is ultimately rooted in the laws of chance and probability and in the second law of thermodynamics. In contrast, the parts of our body are not unorganized at all. Although they do follow the laws of physics, they also exchange information by sending sensory data to the brain. There the sensory data are compared to our intended position in space, and corrective impulses are send to the body parts (if needed). Once we are dead the information flow stops, no more corrective action is taken, and the laws of physics take over.
Classical Physics vs the Study of Systems: Classical physics basically tried to break down natural phenomena into an interaction of elementary units that were governed by "blind" laws of nature. Other sciences, e.g. biology, followed this mechanistic approach and tried to resolve life phenomena into atomic entities and partial processes. The living organism was resolved into cells, its activities into physiological and ultimately physicochemical processes, behavior into unconditioned and conditioned reflexes, the substratum of heredity into particulate genes, and so forth. Although this level of understanding tells us much about the parts, it tells us little about the behavior of the whole. If we really want to understand, say a tree, the atoms and molecules that compose it have to be understood at an organismic level. We have to understand the organization and dynamic interactions of the parts, in order to understand the whole (the whole is more than the sum of its parts). In short, we have to understand the tree as a system of interrelated parts (for example, leaving out the stimuli exchange between sensors, brain, and muscles would severely diminish our understanding of the body as a whole). The same applies to understanding the Earth, the Universe, and any other complex entity.
In a most general sense, a system is any portion of the material universe which we choose to separate in thought from the rest of the universe for the purpose of considering and discussing the various changes which may occur within it under various conditions (J. W. Gibbs).
Principally there are two types of systems, open systems and closed systems.
 |
 |
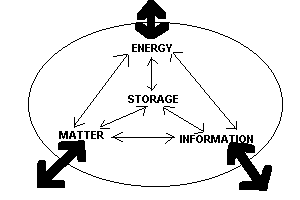
| Open systems exchange energy, matter, and information with the surrounding world. Energy, matter, and information can enter or leave the "oval" system. While inside the system, they can change form, interact with each other, or go into and out of storage. The earth can be considered as such a system. For example: energy enters the system, interacts with matter on the earth, can be stored as plant tissue, and can leave as the earth radiates heat back out to space. |
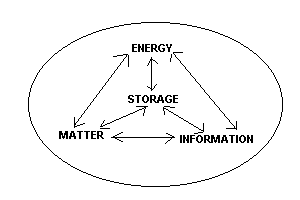
Closed systems are self-contained and do not interact with their surroundings. Energy, Matter, and Information are exchanged and altered only within the system (oval), nothing enters, nothing leaves. Energy, matter, and information can be placed into and taken out of storage, but nothing can leave the "oval" system. An example would be the earth itself in terms of matter. There are lots of interactions taking place within the earth system, but very little matter leaves or enters. |
Outputs from systems can feedback into the system and influence how the system responds to information, energy, or matter. The paths taken by the information, energy, or matter are called feedback loops. If a change in a system feeds back into the system and results in further change in the same direction, positive feedback has occurred. If a change in a system results in change in the opposite direction, then negative feedback has occurred. Example 1: during the cold season, snow may fall on the ground, which with its bright, reflective surface, will reflect more of the sun's energy back into space, less heat remains on the Earth surface. Thus cold conditions lead to more cold, we have a positive feedback loop. Example 2: when air cools, it looses its ability to hold moisture, i.e. relative humidity goes up. If it cools enough to reach 100% humidity, condensation will occur and water changes from the vapor to the liquid state. The latent heat of condensation starts to heat up the surrounding air and works against (negates) the initial downward trend of the system temperature. Thus, initial cooling leads to subsequent temperature increase and we have a negative feedback loop.
All systems are dynamic and ever changing, but as long as there is some internal regulating mechanisms they will be in some sort of equilibrium state. For the description of the Earth system we are concerned with two types of equilibrium states, steady-state equilibrium and dynamic equilibrium.
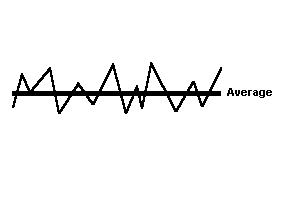 |
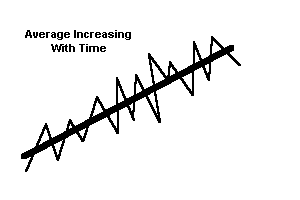 |
| Illustration of
steady-state equilibrium. For example, with respect to energy, the Earth can be considered to be in steady-state equilibrium. It gains about as much energy as it looses in any particular year, and it stores about the same amount of energy from year to year. Its energy fluctuates around an average. |
Illustration of
dynamic equilibrium. A value fluctuates around an average, but the average changes with time. The Earth's climate may exhibit this behavior. Taken over a long period of time, it appears that the Earth's average temperature fluctuates, and is in a state of dynamic equilibrium. |
 |
Another property of many natural systems, although not necessarily self-evident, is that they are self-organizing by their very nature. For example, take a chain made out of paper clips. The mere presence of the chain suggests that someone (a creator) has taken the trouble to link paper clips together to make a chain. You would not think that it is in the nature of paper clips to organize themselves into chains. |
| But, assume for a moment that the paper clips are all slightly opened up (by nature), and if you then shake them around in a cocktail shaker, you will find after a while that the clips have organized themselves into chains of variable length. These chains are not as neat as chains put together by hand, but they are chains nonetheless. Continued shaking will not bring back the original state (single clips). Thus, once something has happened, it does not un-happen so easily. It is this asymmetry that is the basis of self-organization. | |
Finally, the dynamic systems that we will be concerned about in this lecture are self-regulating. This means that the system is able to maintain its essential variables within limits acceptable to its own structure in the face of unexpected disturbances (W.B. Cannon, 1929-32). Basically, we are looking at a process of interaction or mechanism which balances various influences and effects so that a stable state or a stable behavior is maintained. Example: the size of the pupil of the human eye is negatively correlated (negative feedback loop) with the intensity of light entering the retina. This keeps the amount of light within the limits of optimal processing of visual information. Too much light would destroy the light sensitive cones of the retina. The blood sugar content and many other chemical quantities are similarly balanced within the human body. This self-regulated dynamic equilibrium is also referred to as homeostasis.
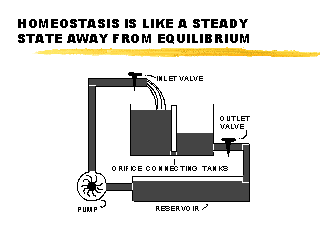 |
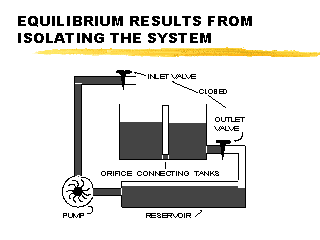 |
| Homeostasis can be illustrated by a partitioned tank with connected partitions. Water moves between the partitions, and it moves faster when the water level difference increases (increase in pressure differential). This state of affairs can only be maintained if we constantly recycle water into one partition with a pump. At any given pump rate there will be an elevation difference that produces a flow between the partitions that equals the pump rate. Although the water levels will be stable at a given rate, this is not an equilibrium state. The unequal fluid levels can only be maintained as long as the pump is running. This non-equilibrium steady state is called homeostasis. |
Once the pump is turned off, the excess water from the left half of the tank will drain into the right half until the two fluid levels are equal (at equilibrium). Thus, our steady state system (at left) would return to equilibrium once we turn off the pump. |
Another example of dynamic self-regulation is the temperature control of the human body. A total of five processes are involved in body temperature regulation: biochemical heat production (slow combustion/oxidation of sugar in our cells) is the main source of heat; and heat output can be increased by shivering (core and skin modulated, muscles burn more fuel), whereas sweating (evaporation of water on the skin) and increased circulation through bloodvessels under the skin helps to rid the body of heat. The figure below shows the range of operation and relative strength of these processes over an ambient temperature range of 40 degrees Celsius.
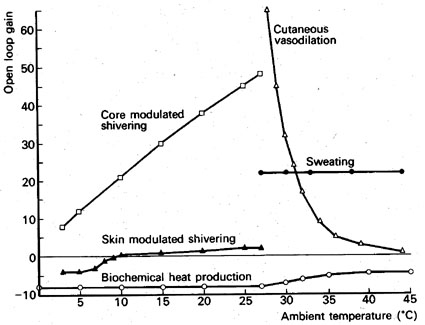
We can link these various processes into a model (each process represented by a mathematical function that is linked to to the others), and examine how it will react as we change ambient temperature. The result is shown in the diagram below.
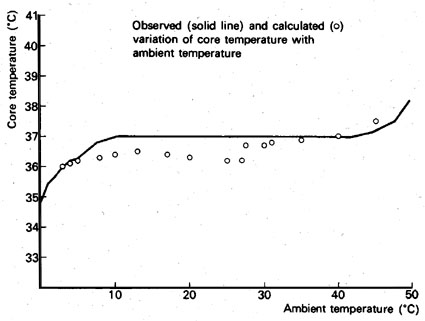
We see that we can describe these body functions and their effect on body temperature in close accordance with observed temperatures. Seeing the body as a system of five interconnected feedback loops adequately represents the reality of human temperature regulation. In the course of this lecture we shall explore how the temperature of the Earth is stabilized within narrow limits and how the biosphere might be involved in that regulation process. For this regulation process the composition of the Earth's atmosphere is crucial, and this is the topic we will discuss next.
Atmospheric Composition
As pointed out earlier, the Earth atmosphere consists mainly of nitrogen (78%), oxygen (21%), argon (1%), carbon dioxide (0.036%), and traces of other gases, such as hydrogen, helium, neon, krypton, xenon, and compounds of those gases (ozone, methane, nitrous oxide, ammonia). The interesting thing about our atmosphere is that its gases at present are not in equilibrium with the rock surface of the planet. If they were in equilibrium they would have fully reacted with the rock sphere and thus oxygen, for example, would not be present at all (it would have formed iron oxides during the weathering of mafic minerals). This, however, is an issue we will explore further when we come to talk about the evolution of the atmosphere. At the moment we will first see what the gases that are currently present are doing with regard to the thermal behavior of the Earth system.
Oxygen: From a chemical perspective, oxygen can be considered the most important gas in the atmosphere. The presence of abundant oxygen allows biochemical processes to proceed with high energy gain, and probably without it highly mobile life forms (like ourselves) would not be possible. Life could go on without oxygen in the atmosphere (it did so for a long time), but at substantially reduced efficiency. Some oxygen is produced in the upper atmosphere by (light induced, photolysis) splitting off of hydrogen atoms from water vapor. Hydrogen escapes from the Earth's gravitational field and leaves oxygen behind. The process, however, constitutes a negligible source of oxygen in the modern atmosphere-biosphere system. Large quantities of oxygen are produced through photosynthesis of plants, and most of it is right away consumed through respiration of living organisms. Because photosynthetic oxygen is split off from carbon dioxide, and the carbon is made into cellulose, carbohydrates etc., burial of plant and animal matter in sediments (removing them from being reoxidized), produces a net gain of oxygen for the atmosphere. This oxygen reacts with reducing substances on the Earth surface (e.g. iron in mafic minerals etc.) and is tied up in solid oxides over time, eventually it would all be used up and the equilibrium atmosphere would contain no more oxygen. Continued photosynthesis and carbon burial keeps the level of oxygen as high as it is, and thus oxygen is in a state of homeostasis, where plant photosynthesis is basically the pump (see homeostasis illustration above), and the rate of burial is the equivalent of the aperture between the two reservoir halves (see homeostasis illustration above). We will get into this in more detail when we talk about the global carbon cycle.
Nitrogen: Nitrogen is a fairly inert gas in the current atmosphere, but although it reacts slowly, its stable form is as the nitrate ion (NO3-), dissolved in seawater. Dissolved nitrogen (in the form of ammonia and nitrate) is essential for living organisms (proteins). When organisms decay, nitrogen is returned to the atmosphere in gaseous form.
Ammonia: Ammonia (NH4-) is another form of nitrogen and is produced by microbes in soils and in the sea. The amount of ammonia produced (1000 megatons per year or more) substantially counterbalances the other acid components (nitric acid, sulfuric acid, carbonic acid) in the ocean-atmosphere system. Only trace amounts are present because it reacts quickly.
Nitrous Oxide: Nitrous oxide (N2O) is produced at a rate of 30 megatons per year by microorganisms in the soil and in the seas. Very little of that shows up in the atmosphere (only trace amounts). It is destroyed by UV light in the upper atmosphere, and gives rise to Nitric Oxide (NO), as well as free oxygen and nitrogen. As nitric oxide it counterbalances ozone production, and it also provides an additional mechanism of returning oxygen and nitrogen to the atmosphere.
Methane: Most methane is produced as a byproduct of bacterial degradation (decay) of organic matter in surface sediments, swamps, and marshlands (about 500 millions tons per year). When methane bubbles up from its source bed and enters the atmosphere it does two things: (1) in the lower atmosphere it combines with oxygen to form carbon dioxide and water, (2) in the upper atmosphere it also combines with oxygen to form CO2 and H2O, but photodissociation of water produces a net gain of oxygen (hydrogen escapes). Because of this, only traces of free methane are present in the atmosphere.
Carbon Dioxide: CO2 is present at a concentration of about 0.036%, and rising. It is the best known of the so-called greenhouse gases. It is produced through respiration by organisms, by burning of forests and fossil fuels, added to the atmosphere through volcanoes, and used by plants as a carbon source in photosynthesis. Cycling of carbon and CO2 is tightly linked to the oxygen cycle. Interaction between these two cycles could be instrumental for keeping the present level of oxygen where it is.
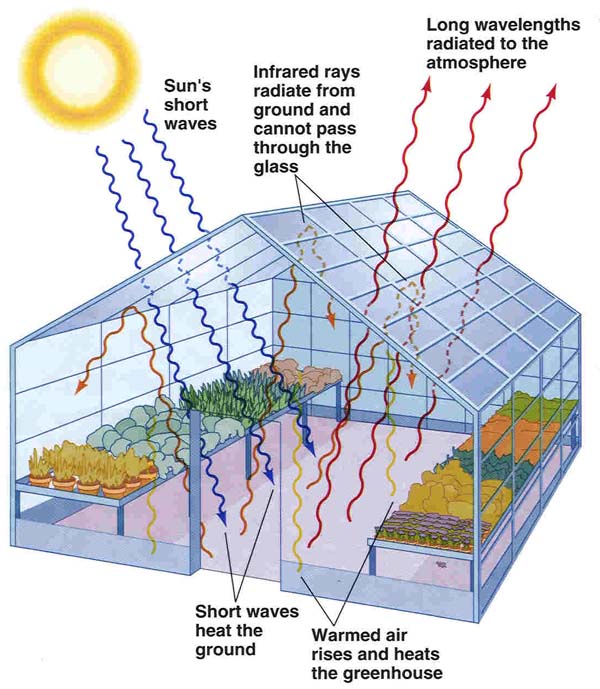
The Greenhouse Effect
The greenhouse effect is a natural phenomenon that is caused by so-called
"greenhouse" gases in the Earth's atmosphere. These gases are more
"transparent"
to the short wavelength radiation (mostly visible "light") from the sun, than to
the long wavelength radiation that is radiated back into space from the Earth's surface.
 |
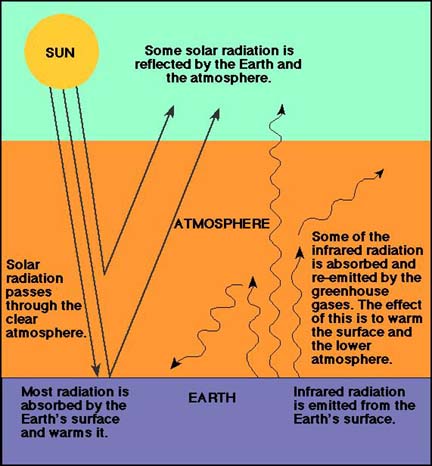 |
| Two diagrams that summarize the greenhouse effect in (a) the name-giving analogue (at left), and (b) as it applies to the Earth's atmosphere (at right). | |
To say that the greenhouse gases trap heat is a bit simplistic. Instead, the greenhouse gases absorb a portion of the infrared radiation that goes back into space, and re-emit it in all directions, meaning that about half of it is returned to the Earth surface. The result of this is that that the global temperature is about 33 degrees Celsius, or 59 degrees Fahrenheit warmer than it would otherwise be. Some of the most important greenhouse gases include carbon dioxide, methane, nitrous oxide, chlorofluorocarbons, and water vapor. Without these gases climatic conditions on Earth would probably be too harsh for most organic life, and the balancing of these greenhouse gases, and with it the regulation of global temperature, is a fascinating scientific question.
Greenhouse gases contribute to this warming effect to various degrees.
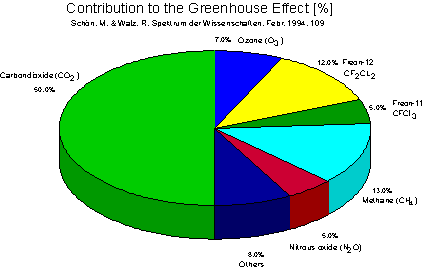
Of the greenhouse gases, carbon dioxide is undoubtedly the best-known, because of the
large amount of it present in the atmosphere. Carbon dioxide occurs naturally, but
its
concentration in the atmosphere has been growing since the onset of the Industrial
Revolution. This increase is due to the combustion of fossil fuels (coal, natural gas, and
petroleum), and can be documented with data from a variety of sources (see below).
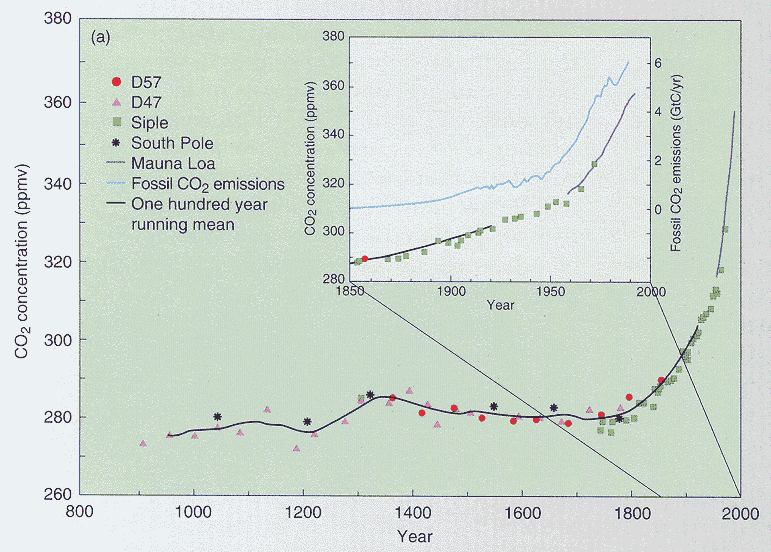
Recent trends in carbon dioxide concentrations in the atmosphere assembled from a
combination of data sources, including direct measurements and measurements of carbon
dioxide in gas bubbles trapped in glacial ice. Notice the extent of agreement among data
types, and the exponential rise in concentrations over the last century.
Another factor that has contributed to the large amount of carbon dioxide in the air is
deforestation. Carbon dioxide is released when organic material is burned or decays. Large
amounts of tropical rainforests have been cleared for agriculture and ranching. These
tropical areas have also been the focus of inefficient logging operations. The United
Nations estimates that during the 1980s, rainforests were destroyed at a rate of 38
million acres per year.
Deforestation and the combustion of fossil fuels has raised the amount of carbon dioxide
in the atmosphere to unprecedented levels. In fact, from the mid-nineteenth century to
1994, there was an increase of more than 25% of the carbon dioxide in the air. This
increase closely matches the growth in carbon dioxide emissions. This increase in carbon
dioxide in the atmosphere is believed by many climatologists to be a cause of an enhanced
greenhouse effect. The other gases that contribute to the greenhouse effect are
present in smaller quantities, but are also important.
Methane
is an extremely effective greenhouse gas, because it is a
more effective absorber of long wave radiation than carbon dioxide. Methane occurs
naturally in the atmosphere as a product of anaerobic decomposition (from places such as
swamps, bogs, paddies, the intestinal tracts of cattle and other animals). The
concentration of methane in the atmosphere has doubled since 1800, probably because of the
increase in human population (as population has risen, so have the number of rice paddies
and cattle).
Nitrous Oxide
is another greenhouse gas whose increase is closely tied to
agriculture. When fertilizers are used to increase crop yields, some of the nitrogen goes
into the air as nitrous oxide. It is also produced in internal combustion engines.
Chlorofluorocarbons
,(CFCs) are manufactured chemicals
with many
uses. They have been used to make foam, cleaners, aerosol sprays, and coolants for
refrigeration and
air conditioning equipment. CFCs have become notorious for their
ozone
destroying capabilities, but are also very effective greenhouse gases. CFCs were not
invented until the 1920s, but they already contribute to the greenhouse effect as much as
methane. CFC atmospheric concentrations are already diminishing as a result of the
Montreal Protocols (an agreement by many nations to discontinue the use of CFC's)..
Water Vapor
is also a greenhouse gas, because it absorbs long wave
radiation that would otherwise escape to space. It exists naturally in our atmosphere and the amount
of it has not risen considerably because of human effects on the environment.
Although carbon dioxide is the most important gas causing the greenhouse effect, trace
gases (methane, nitrous oxide, and CFCs) could collectively double the impact of carbon
dioxide in the future. It has been firmly established that these gases trap the Earth's
long wave radiation and cause a warming effect, and also that the emissions of these gases
has been increasing over time. How these gases interact and how their respective
cycling through the atmosphere affects world climate, however, is a complex issue that
leaves room for uncertainty and speculation.
Nonetheless, since the late 19th century, mean surface temperatures has risen 0.3
- 0.6 degrees Celsius (see below) and thirteen of the hottest years in over a
century were between 1980 and 1992.
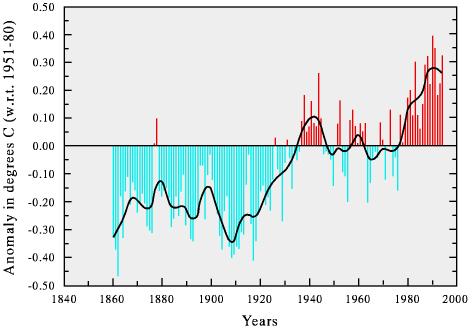
Warming trend of world climate.
In a recent article in GSA Today by David Gutzler, data are presented that show that current global temperatures are warmer than at any time during the past millenium, suggesting that the observed warming trend is at least in part due to anthropogenic input of atmospheric greenhouse gases.
Satellites also show a shrinking snow cover in the Northern Hemisphere.
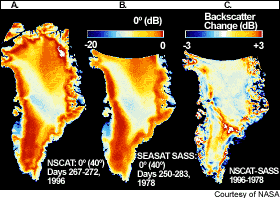 |
Satellite radar backscatter from the
Greenland icecap has changed over the last two
decades and reflects a reduction in the snow cover and an increase of more reflective icy
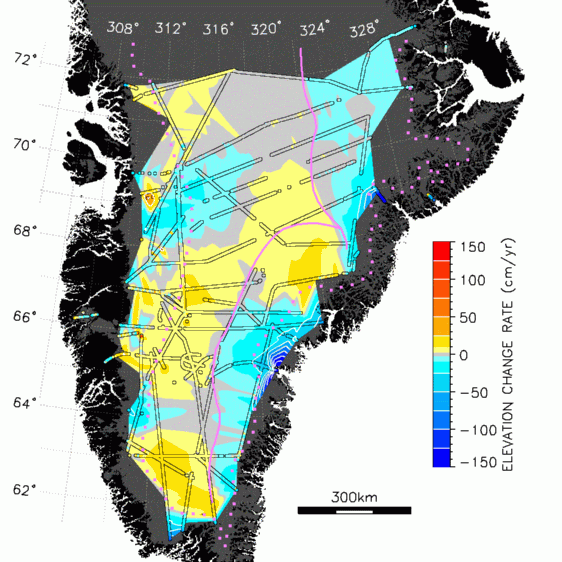
surfaces. Areas with increased radar backscatter are melting. These findings compare well with elevation measurements of the ice surface (picture below). The areas of largest elevation drop (ice volume loss) coincide with the areas in the left picture that show an increase in backscatter (brownish) over the last 2 decades (the third image that shows backscatter change). |
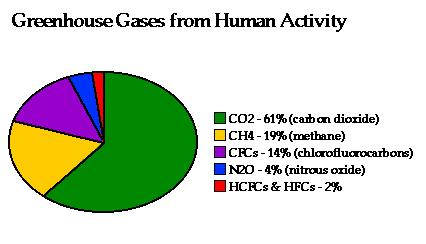
Proportions of anthropogenic greenhouse gas emissions.
If greenhouse gas emissions continue to grow at projected rates, computer models
predict that there will be an increase in temperature 1.5 - 5.5 degrees Celsius between
1990 and 2100. A temperature increase this large would come close to
equaling the amount
of warming that has taken place since the last ice age 18,000 years ago. According to most
of the studies done by the Inter-governmental Panel on Climate Change, "the observed
warming trend (see above) is unlikely to be entirely natural in origin". The IPCC
points out that their ability to understand the extent of human influence on climate
change is limited due to the "noise of natural variability", but also states
that "the balance of evidence suggests that there is a discernible human influence on
global climate"..
The issue of global warming is extremely complex and involves many inter-relationships
between greenhouse gases carbon cycling, and other related (linked) geochemical cycles
(calcium, oxygen, phosphorous, nitrogen) on Earth. Although studies show that the recent
warming trend is likely due to human intervention, there is still much more research to be
done. Human decisions about greenhouse gas emissions may well determine the future of
world climate.
Chapter 8