EUAN NISBET
Euan Nisbet is in the Department of Geology, Royal Holloway, University of London, Egham, Surrey TW20 0EX, UK.
There is evidence of a variety of early organisms from the Archaean — some 4,000 to 2,500 million years ago. Now life at deep-sea hot springs can provisionally be added to the list.
"Beginnings; are apt to be shadowy" wrote Rachel Carson[1]. So it is with the early record of life. In the past few years, plenty of clues have emerged, both from geological studies and inferences drawn from the molecular biology of modern organisms. Often the veil of shadows has lifted a little, only to show more darkness behind. Yet, bit by bit, speculation as to the nature and ecology of early life is being based on a firmer footing.
Rasmussen (page 676 of this issue[2]) is the latest to tweak the veil — he reports evidence of early microbial life in deep-sea volcanic rocks that are some 3,200 million years old. The evidence is thread-like filaments, twining and twisting in different directions, that may be fossils of microorganisms. The filaments occur in a volcanogenic massive sulphide. This is a type of metal deposit that is usually formed on the sea floor, at depths far below the light-penetration zone, and at temperatures near or above the limits tolerated by life.
Rasmussen interprets the filaments as the remains of subsurface prokaryotes, microbes such as bacteria that inhabited environments beneath the sea floor. The implication is that the organisms were chemotrophic hyperthermophiles — that is, non-photosynthetic organisms, which used inorganic matter in their environment as an energy source, and which lived at or near 100 °C. Prokaryotes spread readily: if Rasmussen's interpretation is correct, they would have been widespread in the deep-water volcanic systems of the time, living in and around hydrothermal systems (submarine hot springs).
The ecological map of the Archaean aeon, the time from around 4,000 million to 2,500 million years ago, is gradually becoming less vague. There is widespread evidence for photosynthetic life (both anoxygenic and oxygenic) before 3,000 million years ago, and possibly from as early as 3,500 to 3,700 million years ago[3]. There is now molecular fossil evidence for the existence of cyanobacteria 2,700 million years ago[4, 5]. Similarly, data on ancient carbon and sulphur isotopes[6, 7] support the deduction that methane-generating organisms — methanogens — recycled dead organic matter in sediment; and also that a diverse, hydrothermal-associated, microbial community of chemotrophs existed in waters of shallow and medium depths. There has been speculation that non-photosynthetic Archaean organisms lived in or around hydrothermal systems in deeper waters. But until the findings of Rasmussen[2] that view rested mainly on inference and on the surmise that certain deposits, interpreted as being biological in origin, formed below the base of wave action.
Physical models of the late Archaean Earth point to a variety of habitats for life (Fig. 1). In shallow coastal waters, there were microbial-mat communities, built by cyanobacteria, which carried out oxygenic photosynthesis. In muds and lower parts of the microbial mats, anoxygenic photosynthesizers and methanogens were probably present. Photosynthetic plankton were probably abundant in the ocean surface waters.
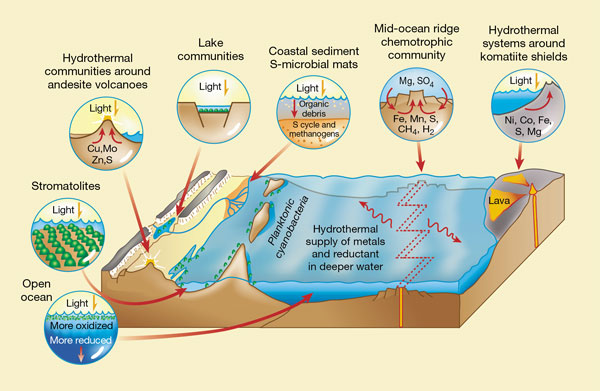
Figure 1 A map of Archaean ecology — places where early life may have flourished. Among such settings
are mid-ocean ridges (chemotrophic communities, including hyperthermophiles); hydrothermal systems
around so-called komatiite shields and andesitic island-arc volcanoes (mixed chemotrophism and
photosynthesis); shallow waters (oxygenic and anoxygenic photosynthesis); the open ocean
(photosynthetic plankton); and muds in the sea floor (methanogens and exploitation of the sulphur cycle).
New findings2 also point to deep-water hydrothermal systems as habitats for life. In each habitat depicted
here, the red arrows show fluid movement. The atmosphere at this time would have been rich in CO 2 and
N2, with minor amounts of NOx, SO x and CH4.
Rubisco is the enzyme responsible for carbon dioxide fixation in photosynthesis — isotopic evidence6 implies that rubisco-based bacteria directly handled about a fifth of the carbon flux emitted from the Earth's interior, as today, and by doing so managed the global carbon cycle. For life to be productive enough to manage carbon, it is likely that modern-style sulphur and nitrogen cycles were also in operation.
Non-photosynthetic habitats, where sulphur chemistry could be exploited, were probably also widespread[7, 8]. Here, the communities could have depended on the contrast between relatively oxidized water and relatively reduced hydrothermal fluids, or they might have used reduced organic debris. In particular, hydrothermal microbial communities may have lived around mid-ocean ridges, volcanic ocean islands and island-arc volcanoes. In the mid and late Archaean, when oxidation power in the surface water was available from surface photosynthesis, such communities may have been prolific. Before the evolution of photo synthesis, the oxidation contrast would have been less. But it would nonetheless have been present, supplied as seawater sulphate.
The work of Rasmussen[2] and of others such as Roger Buick adds a new realm — predicted, but previously only surmised — to the map of Archaean ecology. Moreover, although Rasmussen's work does not show that deep-water hydrothermal life came before photosynthetic life, it does lend circumstantial support to the argument that steps in the early history of life took place around hydrothermal systems. Analysis of ribosomal RNA in modern organisms allows evolutionary trees to be constructed, and inferences to be drawn from them. The 'standard' interpretation of this line of evidence8 is that chemotrophic hyperthermophile communities preceded the advent of photosynthesis. Perhaps, then, the earliest Archaean life was confined to the vicinity of hydrothermal systems.
Several implications follow from this possibility. Much of life's 'housekeeping' chemistry might have evolved in such settings, where metal sulphides were common and the danger of heat shock was ever present[9]. So, for example, proteins incorporating nickel may reflect their origin near high-temperature lavas (known as komatiites), which can contain nickel sulphide deposits; the use in proteins of copper, zinc and molybdenum (metals typical of hydrothermal deposits) could likewise record their heritage. Even the role of heat-shock proteins as chaperonins, which have a crucial part in assembling complex proteins, may date from the hazardous environment near hydrothermal systems.
Conceivably, sensitivity to temperature may also have developed around deep-water hydrothermal systems. This may have preadapted organisms to use light: spreading into shallower habitats, microbial mats may have exploited first anoxygenic then oxygenic photosynthesis in developing ecosystems of progressive complexity[10].
Here we are deep into guesswork, however, and there are difficulties with the evidence. Disputes surround the interpretation of data provided by ribosomal RNA[11]. And the history of the sulphur cycle is puzzling. Conventional isotope work suggests that there is little isotopic fractionation of sulphur in Archaean sediments, although such fractionation would be expected if complex sulphur cycles had evolved. Yet, from the molecular evidence it seems that the sulphur cycle, and by implication sulphur fractionation, is of great antiquity. However, recent high-resolution studies7 show a wide range of isotope ratios in well-preserved sediments 2,700 million years old.
The 'sulphur problem' may be related to the movement of fluid and sediment by multicellular organisms. Although there is geological evidence suggesting that ancestors to such organisms were present in the Archaean[4], it may have been much later before they were physically able to control redox zones in microbial mats and produce regions of fractionated sulphur large enough to be sampled by conventional techniques.
Finally, the reasons for the rise in molecular oxygen, which seems to have occurred in the early Proterozoic some time before 2,000 million years ago, remain unclear: that rise may have been linked to internal controls in the system incorporating rubisco, mitochondria and chloroplasts[12, 13]. And we can only guess about the history of predation.
The study of Archaean rocks is like a forensic investigation based on heavily smudged fingerprints. Rocks can be altered, whereas textures (such as reported by Rasmussen2) can be misinterpreted, and chemical and isotopic tracers[4, 5] can be of later origin. Yet, for all the uncertainties, the map of life's early evolution grows ever more detailed as new terrains are inked in. But, as Roger Buick has remarked, the map is still medieval in aspect, with large blank areas marked 'Terra incognita', or 'Here be cannibals'. And we still know little about the far edge of the world of life — where did it come from?
References
1. Carson, R. The Sea Around Us (Penguin, London, 1956).
2. Rasmussen, B. Nature 405, 676-679 (2000).
3. Rosing, M. T. Science 283, 674-676 (1999). Links
4. Brocks, J. J. et al. Science 285, 1033-1036 (1999). Links
5. Summons, R. E. et al. Nature 400, 554-556 (1999). Links
6. Schidlowski, M. & Aharon, P. in Early Organic Evolution: Implications for Mineral and Energy Resources (ed. Schidlowski, M.) 133-146 (Springer, Berlin, 1992).
7. Grassineau, N. et al. Terra Abstr. 4, 261 (1999).
8. Stetter, K. O. in Evolution of Hydrothermal Ecosystems on Earth (and Mars) (eds Bock, G. R. & Goode, J. A.) 1-18 (Wiley, Chichester, 1996).
9. Nisbet, E. G. & Fowler, C. M. R. Geol. Soc. Lond. Spec. Publ. 118, 239-251 (1996).
10. Nisbet, E. G. & Fowler, C. M. R. Proc. R. Soc. Lond. B 266, 2375-2382 (1999).
11. Doolittle, W. F. Sci. Am. 72-77 (Feb. 2000).
12. Joshi, H. M. & Tabita, F. R. Proc. Natl Acad. Sci. USA 93, 14515-14520 (1996). Links
13. Tolbert, N. E. in Regulation of Atmospheric CO2 and O2 by Photosynthetic Carbon Metabolism (eds Tolbert, N. E. & Preiss, J.) 8-33 (Oxford Univ. Press, 1994).