
click to expand
click to expand
Overview of an article in Volume 98 pages 815-819 of Proceedings of the National Academy of Science, USA. on January 30, 2001.
Mountain View, Calif.-- Duplicating the harsh conditions of cold interstellar
space in the laboratory, scientists from The Astrochemistry Laboratory at NASA's
Ames Research Center and the Department of Chemistry and Biochemistry at The
University of California Santa Cruz have created chemical compounds that may
have been important for life's origin.
In their laboratory, this team regularly make copies of the extremely cold ice
particles that make up dense interstellar clouds. These clouds are the
birthsites of stars and star systems and the smoke sized ice particles are the
building blocks of the planets, asteroids, meteorites and comets which orbit the
stars. Even after they are formed, planets continue to collect material from the
debris (dust, asteroids, meteorites, and comets) from the star formation
process, and life on Earth is thought to have emerged from this primordial
chemical soup.
The most common scientific theory for the origin of life on Earth is that
somewhere in the vast chemical resources available on the early Earth,
conditions favored the formation of chemical compounds and chemical processes
which eventually led to life. In contrast, this new work shows that the early
chemical steps believed to be important for the origin of life do not
necessarily require an already formed planet to occur. Instead, they can readily
be taken in the depths of space long before planet formation occurs. This
implies that the vastness of space is filled with compounds which, if landing in
a hospitable environment, can help jump-start the origin of life.
The main ingredients of interstellar ices are familiar, simple chemicals frozen
together. Mostly water, they also contain some ammonia, carbon monoxide, carbon
dioxide and the simplest alcohol, methanol. The NASA Ames - University of
California Santa Cruz team freezes a mixture of these chemicals into a thin
solid ice at temperatures close to absolute zero (-441°F, / -263°C) under
extreme vacuum conditions. This ice is then exposed to harsh ultraviolet
radiation that mimics the radiation in space produced by neighboring stars. It
has been known for a long time that ultraviolet irradiation of icy solids
produces chemicals more complex than those originally present in the ice, and
there was speculation that some of these chemicals might have played an
important role in early Earth chemistry. However, the recent development of
Exobiology and Astrobiology as interdisciplinary research fields has brought
together astronomers, biologists and funding opportunities in a way that wasn't
possible ten years ago.
"We started this work motivated to find the types of compounds that might
be in comets, icy planets and moons, providing guidance for future NASA
missions," space scientist and team leader Lou Allamandola said.
"Sure, we expected that ultraviolet radiation would make a few molecules
that might have some biological interest, but nothing major. Instead, we found
that this process transforms some of the simple chemicals that are very common
in space into larger molecules which behave in far more complex ways. Ways which
many people think are critical for the origin of life, the point in our history
when chemistry became biology," he continued.
"Instead of finding a handful of molecules only slightly more complicated
than the starting compounds, hundreds of new compounds are produced in every
mixed ice we have studied," space scientist Scott Sandford said. He
continued, "We are finding that the types of compounds produced in these
ices are strikingly similar to many of those brought to Earth today by infalling
meteorites and their smaller cousins, the interplanetary dust particles. Every
year more than a hundred tons of extraterrestrial stuff falls on the Earth, and
much of it is in the form of organic material. In the early life of our solar
system, before the debris from its formation was fully cleared away, these
materials were deposited on the Earth in far greater quantities than we see
today. Thus, much of the organic material found on the Earth in its earliest
years probably had an interstellar heritage."
"A number of years ago I found that some of the extraterrestrial organic
compounds brought to Earth in the Murchison meteorite could form membranous
vesicles when they interacted with water," said team member Dave Deamer,
Professor of Chemistry at the University of California at Santa Cruz. Vesicles
are microscopic, hollow droplets with sizes, shapes and structures similar to
those of certain living cells. "All life today is cellular, and cells are
defined by membranes that separate the cytoplasm from the outside world. When
life began, at some point it became compartmented in the form of cells. But
where did the first cell membranes come from? Maybe they were composed of
molecules similar to those we discovered earlier in meteorites," Deamer
continued. "When I learned of the ice experiments at NASA Ames, I went to
the Astrochemistry Lab intending to find out what would happen when their
complex organic mixtures were allowed to interact with water. To our surprise
and delight we found that vesicular structures formed that looked very much like
those we saw in the Murchison material."
"We now know that of the hundreds of new compounds we make in these
interstellar ice simulation experiments, many have properties relevant to the
origin of life," said biochemist Jason Dworkin. "Upon the addition of
liquid water to the organics produced during ice irradiation, some of these new
compounds, with no outside help, organize themselves into tiny vesicles with
complicated structures. Other new compounds formed are so much more complex than
what we started with that they glow when exposed to UV light. Not only that, but
these molecules, which can convert energy from the ultraviolet light to the
visible range, become part of the self-formed vesicles," continued Dworkin.
"Molecules that do these things are thought to be extremely important for
the origin of life. Membrane structures are necessary to separate and protect
the chemistry involved in the life process from that in the outside environment,
and all known biology uses membranes to capture and generate cellular
energy," Dworkin said.
Sandford added, "The ready formation of these and other biologically
interesting compounds by irradiating realistic simple interstellar ices shows
that some of the organics falling to Earth in meteorites and interplanetary dust
particles might have been born in the coldest regions of interstellar space, and
that delivery of these compounds by comets, meteorites and interplanetary dust
particles could well have been important in the origin of life on Earth."
Allamandola concluded, "The spell is now breaking that interstellar
chemistry is a chemistry of relatively small and simple molecules. We are just
now beginning to realize that we are only seeing the tip of the iceberg in terms
of extraterrestrial molecular complexity. Since these experiments were designed
to simulate the conditions in all dense molecular clouds, the birthsites of new
stars and planetary systems, very complex organic molecules that might be
important for the origin of life could well be falling on the surfaces of newly
formed planets everywhere in the universe. For example, of the things which
bring extraterrestrial chemicals to the Earth, comets are thought to be closely
related to interstellar ices. I know I hold a minority view on this nowadays,
but I suspect that even deep inside a comet, which is mainly water ice after
all, reactions are much further along than we think and the chemistry quite
complex."
Their results are published in the current, special Astrobiology issue of the
"Proceedings of The National Academy of Sciences, USA". The authors
include Drs. Dworkin, Sandford, and Allamandola of NASA's Ames Research Center
and Professor Deamer of the Chemistry and Biochemistry Department of the
University of California, Santa Cruz.
This research is supported by the Space Science Division at NASA Ames Research
Center and the Offices of Exobiology and Astrobiology at NASA Headquarters,
Washington, D.C.
 |
These droplets (~10 µm across) show structures reminiscent of cells (although they are not alive). They are from a chemically separated fraction of the bulk residue. | ||
click to expand |
|||
| These droplets (small ones are ~10 µm across) glowing under black light in the microscope show internal structure and suggest chemical complexity. They are from a chemically separated fraction of the bulk residue. | |||
click to expand |
|||
 |
|||
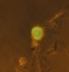
| This is a vesicle (~10 µm across) glowing under black light in the microscope made from the bulk residue. Proof that it is a hollow vesicle, rather than a simple drop of oil, is the green pyranine dye which we have trapped inside of it. | |||
click to expand |
The Astrochemistry Lab at NASA Ames
The equipment used to simulate space (center image)
Related material in the July 1999 issue of Scientific American
Dr. Jason P.
Dworkin
jdworkin@mail.arc.nasa.gov
(650) 604-0789
Prof.
David W. Deamer
deamer@hydrogen.ucsc.edu
(831) 459-5158
Dr. Scott A.
Sandford
ssandford@mail.arc.nasa.gov
(650) 604-6849
Dr. Louis J.
Allamandola
lallamandola@mail.arc.nasa.gov
(650) 604-6890