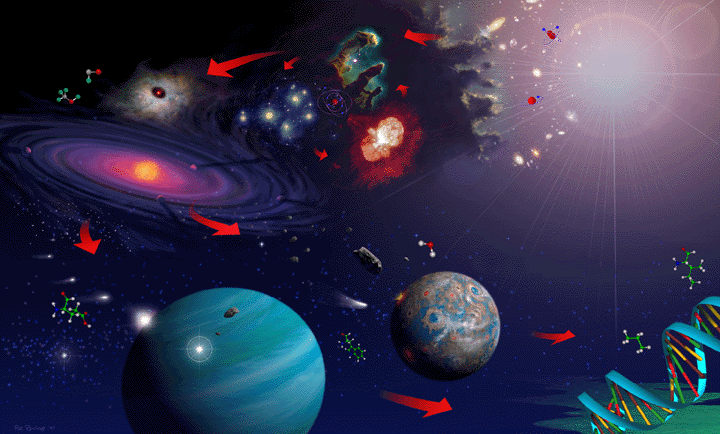
The image above shows an artist's rendition of a flow of events in a 13-billion year history of the Universe from the Big Bang at upper right counter-clockwise to the formation of life on Earth at lower right.
Chapter 10: The Origin of Life and its Consequences

The image above shows an artist's rendition of a flow of events in a 13-billion
year history of the Universe from the Big Bang at upper right counter-clockwise to the
formation of life on Earth at lower right.
The assembly of simple organic molecules into a metabolizing, self-replicating, and membrane-bound form that we might call life has so far eluded experimental approaches. W. H. Schlesinger
When the Earth formed at about 4.6 to 4.5 billion years ago, it was initially a cold place bombarded by meteorites, and later on it probably got so hot that the surface may even have been in a molten state (the magma ocean). Neither condition was very conducive to life as we know it. Yet a billion years later we have rocks that contain evidence (fossils, stromatolites) of microorganisms such as bacteria and blue-green algae.
|
|
| Stromatolite domes along the shoreline of an ancient sea | Some pictures of the earliest microorganisms found on Earth. They resemble filaments of modern blue-green algae (cyanobacteria). |
What happened between the time when the magma ocean gave way to solid crust (including an early atmosphere and ocean), and the time we find earliest evidence of life in the form of fossils? Considering that rocks get continually recycled through Earth history, and that as a result only very small quantities of the earliest rocks are preserved (and these are highly altered and metamorphosed ), it should come as no surprise that the earliest beginnings of life are enigmatic. Basically, scientists do not know the exact processes that led to life on Earth. Because the evidence for very primitive life probably was destroyed by more efficient life forms that succeeded them, we may never know the exact answer. Nonetheless, scientists have made important progress in understanding the types of chemical processes that may have led to living structures.
To illustrate the problem, just imagine the following: A friend that you know to live in your hometown of Chicago, calls you and tells you that he currently is in San Francisco. How would you know the exact route he took to get there? He could tell you of course, but lets assume that he won’t. From the time of your last encounter with him you might be able to guess whether he traveled from Chicago by airplane, train, or car. Then you could try to guess which airline or train he took, or which highways he might have followed. However, unless you get access to the reservation system of the airline or travel agency that booked his flight or train, or to the computer of his credit card company to track his movements, or even follow his suspected route and check for eyewitnesses that might have seen him, it will all remain just that -- an educated guess. Unless you have this detailed information, you have to be satisfied with developing plausible scenarios based on (1) your knowledge of the starting and ending points and (2) the approximate time it took him to make the trip.
We are in a similar predicament with our understanding of the origin of life. Because we don't have detailed information on the exact steps we will have to be content with developing plausible scenarios based on information concerning conditions on the early Earth around the time life originated nearly four billion years ago. It is these scenarios that we will discuss in the following paragraphs.
Advanced forms of life existed on earth at least 3.55 billion years ago, because in rocks of that age we have found fossilized imprints of microorganisms that look very much like modern blue-green algae (cyanobacteria).
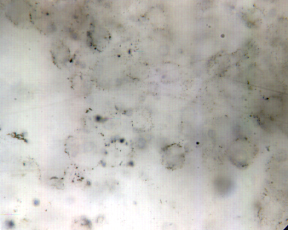 |
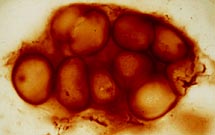
Early cellular remains: These microspheres are from the Barberton Greenstone Belt (South Africa) and have an age of 3.4 billion years. They are quite small, generally less than 10 microns. |
 |
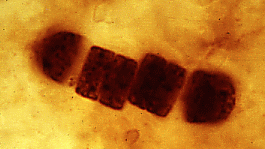  |
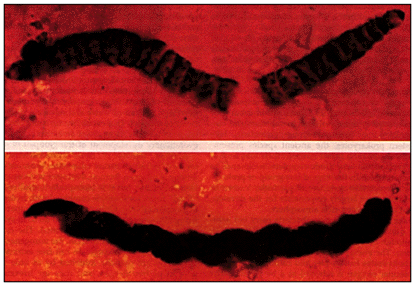
| Precambrian microfossils: At left we have filaments that probably represent blue-green algae (Bitter Springs Formation, Australia, about 850 million years old). At right we have a closeup of another cyanobacterial filament (top; Palaeolyngbya), and a closeup (bottom) of a colonial chroococcalean form. | |
Organic life probably existed even earlier than that, based on carbon isotope data from the 3.8 billion year old Isua Formation of Greenland. The Isua Formation consists of metamorphosed sediments that contain graphite with an unusual carbon isotope ratio. They are enriched in C12 over C13 when compared to carbon isotope ratios in meteorites (presumed to record the primordial carbon isotope ratio of the solar system). Preferential uptake of light carbon (C12) is something that microorganisms routinely do when they absorb carbon from their environment, either by photosynthesis or by metabolizing other carbon compounds. Thus, C12 enrichment in these rocks suggests the presence of living organisms as early as 3.8 billion years ago. Because the earth probably remained inhospitable to life for about 500 million years after its "birth", the time it took to for differentiation, formation of the initial crust, and the formation of a primitive atmosphere and ocean, we have about a 200 to 300 million year time interval within which life had to form and to evolve to the complexity observed in modern bacteria. There is, however an increasing number of observations that give us clues to the Earliest Biosphere and the likely places for Archean life to have obtained a foothold. Recent research has also provided first direct evidence that early life occupied the sites of early seafloor hotspring vents, an observation of particular significance in the light of the discovery of hotspring based life on the modern ocean floor.
| Just to make things more interesting: The carbon isotope data from Greenland (Isua) have come under criticism recently (2005), and there is a need to revisit and reinvestigate this claim of oldest life on the planet. Link to a Nature paper critical of the initial carbon isotope work on Isua. |
This time span was once considered too short for the emergence of something as complex as a living cell. Therefore, a number of people suggested that germs of life may have come to earth from outer space with cometary dust or even via a space probe sent out by some distant civilization. The latter scenario was originally by Francis Crick, one of the scientists that worked out the double-helix structure of DNA, and has more recently been recycled in an episode of Startrek (Star Trek, The Next generation, Season 6, Episode 20). Along with the spontaneous creation that is the basis of many religions, an origin via alien space probe can not be tested and is thus beyond the reach of scientific inquiry. Seeding by cometary dust may not be entirely impossible if it can be confirmed that Martian meteorites do indeed carry evidence of primitive Martian life forms. However, the long journey through the frigid vacuum of space, beginning and ending with a fiery meteorite impact, would surely cause severe degradation of any participating organic materials.
On Earth, the rock record of its earliest history (and thus the history of earliest life) is very skimpy (crustal recycling) and if present has been battered by billions of years of weathering, tectonics, metamorphism, etc. The oldest definitely dated rocks are at Isua, Greenland with an age of 3650-3800 Ma. So far no one has been able to find rocks that date from before 3.8b.y. However, there is a place where material from before that time could have survived essentially unaltered to this day.
Since these proposals of an external origin were made, however, we have accumulated sufficient knowledge to seriously entertain the notion that life on Earth also originated on Earth. If life arose spontaneously by natural processes--a necessary assumption if we wish to remain within the realm of science--chemical considerations suggest that once chemical synthesis started living structures should have arisen fairly quickly. Perhaps even within millennia or centuries, rather than over millions of years.
Prebiotic Chemistry
As pointed out in our Chicago to San Francisco analogy, how this happened is still largely conjectural, though no longer purely speculative. The clues for what might have happened come from the Earth, from outer space, from laboratory experiments, and, especially, from life itself. We know today that the history of life on Earth is written in the bones and shells of fossil organisms, but also in the cells and molecules of existing organisms. Much of what our own DNA is also found in the DNA of other organisms, all the way down to simple bacteria. The common thread that runs through all life is the use of the DNA molecule to store and transmit information. Thus, in order to find out about the origins of life we have to go back to bacteria and beyond. Thanks to the advances of cell biology, biochemistry, and molecular biology, scientists are becoming increasingly adept at reading the DNA record, and also at decoding the relative antiquity of various cell components and metabolic functions (such as oxygen based metabolism, photosynthesis, etc.). As we try to reconstruct the earliest events in life's history we have to assume that all that went on happened without the benefit of foresight (just as our daisies did not purposely cooperate to stabilize Earth’s climate). Every step must be accounted for in terms of antecedent and concomitant events, and must obey the laws of physics and chemistry. Each must stand on its own and cannot be viewed as a preparation for things to come.
The early chemists invented the term "organic" chemistry to designate the part of chemistry that deals with compounds made by living organisms. The synthesis of urea (NH2CONH2) by Friedrich Wöhler in 1828 is usually hailed as the first proof that a special "vital force" is not needed for organic syntheses.
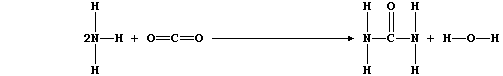
Lingering traces of a vitalistic mystique nevertheless long remained associated with organic chemistry, seen as a special kind of life-dependent chemistry that only human ingenuity could equate. The final demystification of organic chemistry has been achieved by the exploration of outer space. Spectroscopic analysis of incoming radiation has revealed that the cosmic spaces are permeated by an extremely tenuous cloud of microscopic particles, called interstellar dust, containing a variety of combinations of carbon, hydrogen, oxygen, nitrogen and, sometimes, sulfur or silicon. These small molecules would hardly remain intact under conditions on Earth, but would interact to form more stable, typical organic compounds, many of them similar to substances found in living organisms. That such processes indeed take place is demonstrated by the presence of amino acids and other biologically significant compounds on celestial bodies--for example, a meteorite that fell in 1969 in Murchison, Australia, Comet Halley (which could be analyzed during its recent passage by means of instruments carried on a spacecraft), and Saturn's satellite Titan, the seas of which we now know are made of hydrocarbons.

This is a piece of the Murchison meteorite, which fell in Australia. Amino acids found in the meteorite were apparently present in it when it fell.
These compounds do not indicate the presence of life, but rather form spontaneously through normal chemical interactions. Organic chemistry is nothing but carbon chemistry. It just happens to be enormously richer than the chemistry of other elements--and thus able to support life--because of the unique associative properties of the carbon atom. In all likelihood the first building blocks of life arose, as do all natural chemical compounds, spontaneously, according to the rules of thermodynamics.
Actually, as early as the 1930s, Alexander I. Oparin in Russia and J.B.S. Haldane in
England had pointed out that the organic compounds needed for life could not have formed
on the Earth if the atmosphere was as rich in oxygen (oxidizing) as it is today. Oxygen,
which takes hydrogen atoms from other compounds, interferes with the reactions that
transform simple organic molecules into complex ones. Oparin and Haldane proposed,
therefore, that the atmosphere of the young Earth, like that of the outer planets
(Jupiter, Saturn, etc.), was reducing: it contained very little oxygen and was rich in
hydrogen (H2) and compounds that can donate hydrogen atoms to other substances. Such gases
were presumed to include methane (CH4) and ammonia (NH3). Oparin's and Haldane's
ideas inspired the famous Miller-Urey experiment, which in 1953 began the era of
experimental prebiotic chemistry. Harold C. Urey of the University of Chicago and Stanley
L. Miller, a graduate student in Urey's laboratory, wondered about the kinds of reactions
that might have occurred when the Earth was still enveloped in a reducing atmosphere. In a
self-contained apparatus, Miller created such an "atmosphere." It consisted of
methane, ammonia, water and hydrogen above an "ocean" of water. Then he
subjected the gases to "lightning" in the form of a continuous electrical
discharge. After a few days, he analyzed the contents of the mock ocean.
|
|
 |
| The schematics of the Miller-Urey origin-of life experiment. | Stanley Miller with his apparatus. |
Miller found that as much as 10 percent of the carbon in the system was converted to a
relatively small number of identifiable organic compounds, and up to 2 percent of the
carbon went to making amino acids of the kinds that serve as constituents of proteins.
This last discovery was particularly exciting because it suggested that the amino
acids needed for the construction of proteins - and for life itself - would have been
abundant on the primitive planet. At the time, investigators were not yet paying much
attention to the origin of nucleic acids- they were most interested in explaining how
proteins appeared on the earth.
Careful analyses elucidated many of the chemical reactions that occurred in the experiment
and thus might have occurred on the prebiotic planet. First, the gases in the
"atmosphere" reacted to form a suite of simple organic compounds, including
hydrogen cyanide (HCN) and aldehydes (compounds containing the group CHO ). The aldehydes
then combined with ammonia and hydrogen cyanide to generate intermediary products called
aminonitriles, which interacted with water in the "ocean" to produce amino acids
and ammonia. Glycine was the most abundant amino acid, resulting from the combination of
formaldehyde (CH2O), ammonia and hydrogen cyanide.
A surprising number of the standard 20
amino acids were also made in lesser amounts (see below).
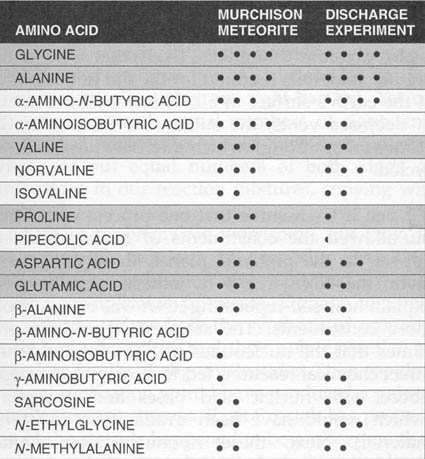 |
A comparison of
amino acids produced in the Miller-Urey discharge experiment
and
amino
acids found in the Murchison Meteorite. The similarities have lent credence to the
idea that the experimental conditions may have approximated the chemistry of the prebiotic
Earth. Since this experiment, many other mixtures of simple gases have been
subjected to various energy sources and have led to similar results. Basically,
under sufficiently reducing conditions, amino acids form easily. In contrast, under
oxidizing conditions they do not form at all, or only in very small amounts. It is an interesting observation in this context, that in living tissues (including our own) most of the chemical bonds are electron-rich (or in a reduced state). Thus, the reducing early atmosphere was a very suitable environment to form these reduced compounds, and may have been essential for the development of early life processes. Our contemporary atmosphere is very electron-poor because of the presence of oxygen - one of the most electron-loving substances. So, deep inside we still have an Archean chemistry, but we live in an oxidizing world. The contrast allows us to have highly efficient and energetic metabolic processes, but it comes at a price. That's why antioxidants, substances that counteract destructive oxidation of body cell matter, are important for our well being. |
In the near future we may expect improved data on organic matter in meteorites from a recent find - the Tagish Lake Meteorite. This bus-sized meteorite blazed to Earth in a spectacular fireball in January 2000 and could have delivered the most pristine primordial matter ever recovered from space and carry important new clues about the origin of life.
 |
Cell membranes are another important component of living cells, they shield the interior (including the RNA/DNA) from harmful changes in the surrounding environment. NASA scientists, while experimenting with organic substances in space conditions, have come up with a possible way in which the raw material for the first cell membranes might have come from outer space. |
The Essentials of Life
Darwin was probably the first to suggest that life could have arisen through
chemistry. In a private correspondence he wrote that life could have originated,
"in some warm little pond, with all sorts of ammonia and phosphoric salts, light,
heat, electricity, etc. present." For much of the 20th century, origin-of-life
research has aimed to flesh out this hypothesis - to elucidate how, without supernatural
intervention, spontaneous interaction of the relatively simple molecules dissolved in the
lakes or oceans of the prebiotic world could have yielded a common ancestor to all living
things on Earth.
Finding a solution to this problem requires knowing something about that ancestor's
characteristics. Obviously, it had to possess genetic information - that is, heritable
instructions for functioning and reproducing - and the means to replicate and carry out
those instructions. Otherwise it would have left no descendants. Also, its system for
replicating its genetic material had to allow for some random variation in the heritable
characteristics of the offspring so that new traits could be selected and lead to the
formation of diverse species.
Scientists have attained more insight into the character of the last common ancestor by
identifying commonalities in contemporary organisms. One can safely infer that intricate
features present in all modern varieties of life also appeared in that common ancestor.
After all, it is next to impossible for such universal traits to have evolved separately.
The rationale is the same as would apply to discovery of two virtually identical
screenplays, differing only in a few words. It would be
unreasonable to think that the scripts were created independently by two separate authors.
By the same token, it would be safe to assume that one script was an
imperfect replica of the other or that both versions were slightly altered copies of a
third.
One readily apparent commonality is that
all living things consist of similar
organic (carbon-rich) compounds. Another shared property is that the
proteins
found in present-day organisms are fashioned from one set of 20 standard amino acids.
These proteins include enzymes (biological catalysts) that are essential to development,
survival and reproduction.
 |
Further, contemporary organisms carry their genetic information in nucleic acids - RNA and DNA - and use essentially the same genetic code. This code specifies the amino acid sequences of all the proteins each organism needs. More precisely, the instructions take the form of specific sequences of nucleotides, the building blocks of nucleic acids. These nucleotides consist of a sugar (deoxyribose in DNA, and ribose in RNA), a phosphate group and one of four different nitrogen-containing bases. In DNA, the bases are adenine (A), guanine (G), cytosine (C) and thymine (T). In RNA, uracil (U) substitutes for thymine. The bases constitute the alphabet, and triplets of bases form the words. As an example, the triplet CUU in RNA instructs a cell to add the amino acid leucine to a growing strand of protein. |
From such findings we can infer that our last common ancestor stored genetic information in nucleic acids that specified the composition of all needed proteins. It also relied on proteins to direct many of the reactions required for self-perpetuation. Hence, the central problem of origin-of-life research can be refined to ask, By what series of chemical reactions did this interdependent system of nucleic acids and proteins come into being?
 |
Anyone trying to solve this puzzle immediately encounters a paradox. Nowadays nucleic acids are synthesized only with the help of proteins, and proteins are synthesized only if their corresponding nucleotide sequence is present. It is extremely improbable that proteins and nucleic acids, both of which are structurally complex, arose spontaneously in the same place at the same time. Yet it also seems impossible to have one without the other. And so, at first glance, one might have to conclude that life could never, in fact, have originated by chemical means. |
The Jump from Primordial Soup to Living Cells
Judging from the Miller-Urey experiment and similar experiments, the primordial ocean might have been a solution that was rich in a wide variety of organic compounds and amino acids, a brew of organic compounds that now carries the popularized name "primordial soup". It is tempting to speculate how, through the process of self-organization, living structures might have emerged from this. The most tantalizing ideas in that direction have to do with DNA and RNA, and are captured in the term RNA-world.
The basic premise of RNA world is this: the first life on earth was based on ribonucleic acids (RNA), a simpler chemical cousin of DNA (deoxyribonucleic acids). RNA can store genetic information and it can catalyze reactions; essential processes in living systems. It has been proposed that RNA, a polymer (long-chain molecule), arose from the gradual stringing together of repeating chemical units, known as monomers, that naturally arose on the primitive earth and were abundant in the "primordial soup".
In support of the view that simple RNA strands formed prior to formation of DNA and proteins (RNA is structurally simpler than DNA, and thus easier synthesized) one can point to the following observations and facts:
Given these observations, it is quite possible that RNA formation and RNA synthesis (and evolution) preceded and initiated protein synthesis. In other words, RNA world is a distinct possibility.
So, how did we get to RNA world?
One hypothesis is that in the primordial soup, ribose (sugar), nucleic acid bases (e.g. adenine, thiamin, guanine, cytosine, uracil), and phosphate joined up to form the first ribonucleotides, that these underwent spontaneous polymerization, and that selection of self-catalytic varieties led to dominance (in the soup) of those primitive RNA types that were able to replicate. The problem is so far that although this is possible in principle, no one has yet come up with a setup where spontaneous reactions produce large quantities of ribose, and we need ribose to put together our RNA molecules.
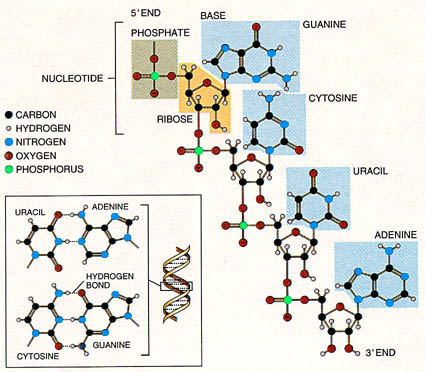 |
| The RNA molecule is composed of so called nucleotides, each of which consist of a phosphate group (green box), the sugar ribose (yellow box), and a nitrogen containing base (blue boxes; guanine, cytosine, uracil, or adenine). Uracil on one RNA strand can pair with adenine on another strand, cystosine can pair with guanine, and we get paired RNA strand in a double helix configuration. Such complementary base pairing is thought to have contributed to the ability of early RNA to reproduce itself. |
Another possibility is that inorganic catalysts were essential for producing the first RNA strands. One possible inorganic catalyst are clay minerals. Recently it has been shown that it is possible to form RNA from monomers on the surfaces of clays, which can catalyze (or chemically assist) the polymerization reaction. Experiments done in test tubes (in vitro) have shown that RNA with one type of catalytic activity can evolve to an RNA with different catalytic properties. These two sets of experiments suggest that it may be possible to demonstrate how clay minerals could have permitted the formation of complex RNA molecules that are capable of evolving in form and function. If this inference is correct, then we may have here a plausible scenario for the origins of life.
Other research along these lines also suggests that polymerization on mineral surfaces may be an important component in the RNA world saga. While many mineral surfaces prefer water and other inorganic substances over organic substances and thus inhibit organic polymerization, certain molecular sieves (artificial inorganic substance) and zeolite minerals (naturally occurring) have 3-dimensional channels where electrically neutral silicon-oxide surfaces absorb organic species in preference over water. It has been proposed that similar materials may have existed on the surface of Earth during its life-origin phase, and that polymer migration along weathered silicic surfaces and micrometer-wide channels in feldspars might have led to assembly of replicating catalytic biomolecules and perhaps primitive cellular organisms.
So, although we don’t know exactly how to get from the primordial soup to RNA world, there are still so many unexplored possibilities that it is much to early to conclude that it simply can’t be done.
Assuming that there is a way to RNA world, the complementary chemistry of base pairing (e.g. guanine - cytosine, adenine - uracil) would very likely have led to the production of complementary copies of RNA, and by repetition of the process to identical copies of the original. This "birth" of the abiotic chemical "replicator" may have been one of the critical steps towards living structures, because it made possible the manufacture of multiple copies of complex molecular structures. Now, to make existing RNA strands to perform this trick is not as simple as it looks. It should theoretically be possible, but as yet we don’t know how to get the process started, or what conditions would be conducive to its spontaneous onset. Again, however, we have to remember that we are still at the beginning of understanding what very possibly could be the quintessence of life itself.
Whichever way we get to RNA world, it very likely was the foundation for protein synthesis, DNA formation, and the emergence of the first cells. Considering the speed at which knowledge of biochemical and molecular biological processes is growing, I suspect that eventually we will find all the pieces of the puzzle. Some obstacles for RNA world
The Impact of Life
Initial life probably was able to utilize the organic molecules that had formed
spontaneously (primordial soup) in order to replicate and to support metabolic functions.
Eventually, however, there must have been so much replication and consumption that
the store of "soup" was gradually depleted. Primitive life might have come
to a halt at that point, had it not been for the
"invention" of photosynthesis.
The first organisms that were lucky enough to have that mechanism available to them
could now utilize solar energy to synthesize sugars, to store energy, and to metabolize
and replicate without being dependent on previously formed organic substances
("soup"). Life probably started to have a major impact on the environment
once photosynthetic organisms evolved.
These organisms started to utilize atmospheric
carbon dioxide, pumped free oxygen into the environment, precipitated calcite due to their
metabolic processes, and thus they began to influence the global carbon cycle that
previously had been purely ruled by chemical equilibrium processes. In that way they
not only began to change the composition of the atmosphere, but they also became a player
in global temperature regulation.
The photosynthetic microbes that remove carbon dioxide from the
atmosphere, however, need more than sunshine to do the job. Certain key
nutrients, especially nitrogen and phosphorous, are needed as well. Because the
amount of nitrogen available to algae in the oceans dictates how much they grow
and how much CO2 they absorb, the carbon cycle is also linked with
the nitrogen cycle. Although some blue green algae have the ability to fix
nitrogen, many others do not. Recently scientists have discovered new
bacteria in the oceans that are nitrogen fixers and may be responsible to
make this essential nutrient available to photosynthesizers.
For a long time, the oxygen produced by photosynthesizers did not build up in the
atmosphere, because it combined with reduced elements in surface rocks (iron, manganese).
This led to the formation of the so called "banded iron formations"
(large sedimentary deposits of iron oxide) and only after all the available iron had been
combined with oxygen, could free oxygen start to accumulate in the air. Once oxygen
had been produced, ultraviolet light split the molecules, producing the ozone UV shield as
a by-product. Only at this point could life move out of the protection of the oceans and
start to colonize the land surfaces. Also, once single celled organisms had evolved,
evolution kept on going, and through billions of years of natural selection gave rise to
the many life forms that we now find in the geologic record. All these issues will
be discussed when we talk about the stages of Earth history in "Earth Systems
History" or "Historical Geology".
 |
To sum up: We have made some progress towards developing a plausible scenario for the origin of life. The needed elemental material was present in the oceans of the early Earth, and simple cells may have developed through a gradual process of organic molecule refinement and aggregation. The oldest fossils that we find indicate that bacteria and blue-green algae had evolved within the first billion years of Earth's history. Over time, cellular structures became more complex, and substantial changes began when photosynthesizing organisms developed and became abundant. The process of oxygen accumulation in the atmosphere was probably slow and took a long time. |